Implant May Replace Monthly Injections for Wet AMD
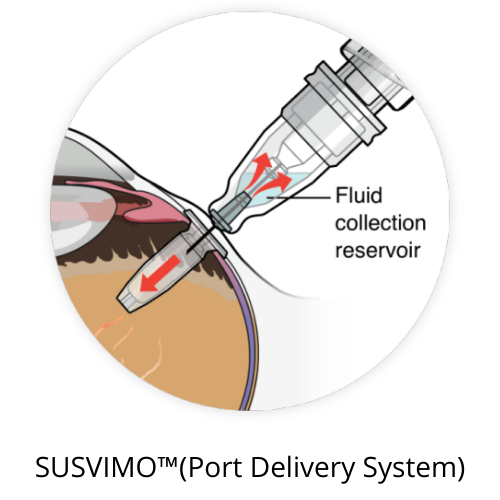

Susvimo™ (Port Delivery System) is now approved for the treatment of wet macular degeneration.
Monthly eye injections for your wet macular degeneration may be a thing of the past. The FDA has approved a device, called Susvimo™, which may significantly reduce the number of treatments needed to treat wet AMD. The implantable refillable device can slowly release medication into the eye and possibly avoid repeated eye injections.
The standard treatment for wet age-related macular degeneration (AMD) is monthly intravitreal injections of anti-VEGF medication. A new FDA-cleared refillable ocular implant that releases the anti-VEGF medication ranibizumab (Lucentis®), and only needs to be refilled every six months may become the new standard of care for wet age-related macular degeneration.
The implant was tested in clinical trials and it showed comparable results to monthly anti-VEGF injections. The Susvimo™ implant is about the size of a grain of rice and must be surgically implanted, but once implanted it only needs to be refilled every six months.
What is Ranibizumab? Ranibizumab is also known as Lucentis®, a type of drug used to treat wet macular degeneration.
The goal was to lengthen the time patients can go without needing treatment inside a clinic and possibly improving vision outcomes as a result of continuous treatment. To be eligible for the implant, a patient must have responded well to at least two anti-VEGF injections.
The implant contains 100 mg/mL of ranibizumab that is slowly and continuously released into the eye with wet AMD. A monthly injection or ranibizumab usually contains 0.5 mg of 10 mg/mL.
About Susvimo™ Wet Age-Related Macular Degeneration
Age-related macular degeneration affects the part of the eye that provides sharp, central vision needed for activities like reading. Wet AMD is an advanced form of the disease that can cause rapid and severe vision loss.
It develops when new and abnormal blood vessels grow uncontrolled under the macula, causing swelling, and bleeding. Worldwide, around 20 million people have wet AMD and it is the leading cause of vision loss in people over the age of 60.
Lucentis® (ranibizumab) was the first treatment found to slow wet AMD, allowing more than 90 percent of patients to keep their vision, according to clinical trials.
But, in the real world, the percentage is closer to 50 percent. And that is because patients are usually undertreated. Most patients with wet AMD have to travel to an ophthalmologist’s office for eye injections every month. This schedule can just be too difficult to maintain for many elderly patients who are struggling with other health issues and are reliant on help to get them to their ophthalmologist visits, so consequently they are undertreated.
An implanted ranibizumab delivery system would allow those patients to get the treatment they require for the optimal outcome without traveling to an ophthalmologist every month for an injection.
Gregory Scimeca, M.D.
Ophthalmologist and Medical Director
The Eye Professionals
Our Locations
